Online purchase of Amoxicillin (Amoxil), where to order, cost.
Pharmacotherapeutic group
Antibacterial agents for systemic use. Beta-lactam antibacterial agents, penicillins.
Broad-spectrum penicillins.
ATX code J01C A04.
Pharmacological properties
Pharmacodynamics
Mechanism of action
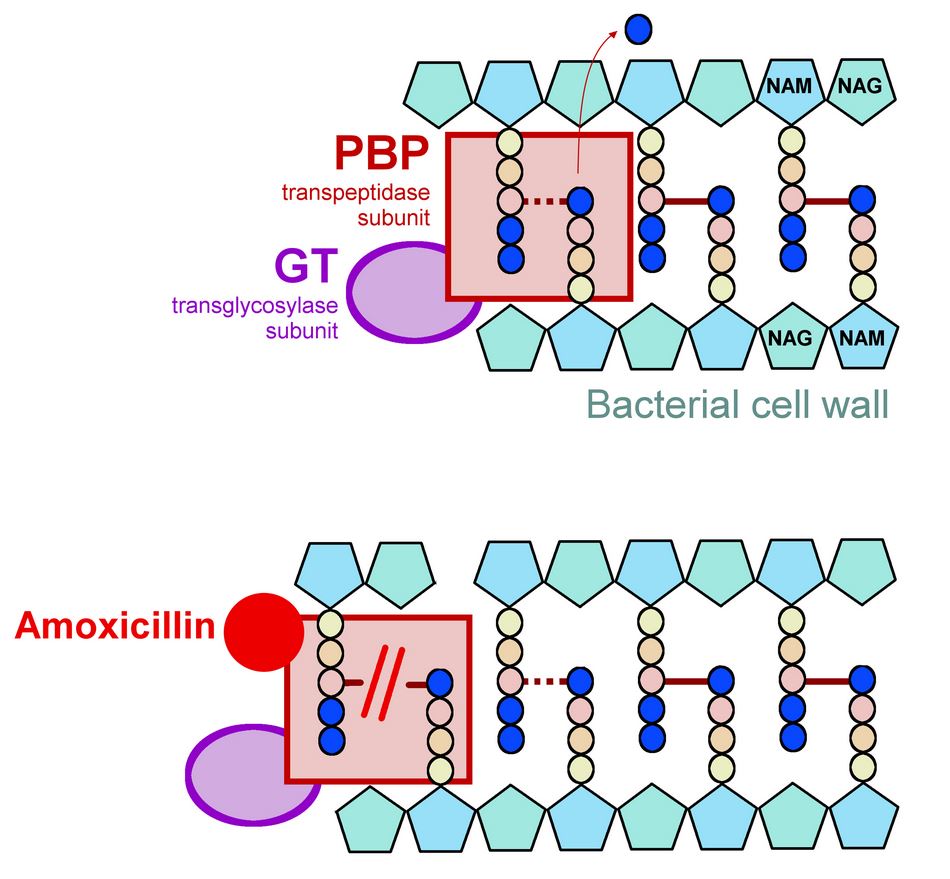
Amoxicillin/Amoxil is a semisynthetic penicillin (beta-lactam antibiotic) that inhibits one or more enzymes (often called penicillin-binding proteins, PBPs) in the pathway for the biosynthesis of bacterial peptidoglycan, which is an essential structural component of the bacterial cell wall. Inhibition of peptide synthesis results in weakening of the cell wall, which is usually accompanied by lysis and cell death.
Amoxicillin is inactivated by beta-lactamases produced by resistant bacteria and therefore the spectrum of activity of Amoxil (amoxicillin) alone does not include organisms that produce these enzymes.
Where to order Amoxicillin (Amoxil) inexpensively?
Cost of the drugIn order to safely and inexpensively buy Amoxil, you have found the best place. Generic Amoxil is a drug based on the active substance (amoxicillin). Antibacterial agent for systemic use. Beta-lactam antibacterial agents, penicillins. Broad-spectrum penicillins. To order the drug over the counter (OTC), you need to follow the link to the Canadian online pharmacy and select the desired dosage and number of pills. The drug is delivered by mail. The cost and quality of our generics will pleasantly surprise you. Buy medicines in our online pharmacy, because this is the best way to save on buying medicines - best price. Also, you do not need an online prescription to buy a generic.
Pharmacokinetic/pharmacodynamic relationships
The time above the minimum inhibitory concentration (T>MIC) is considered the main determinant of amoxicillin efficacy.
Mechanisms of resistance
The main mechanisms of resistance to amoxicillin are:
- Inactivation by bacterial beta-lactamases.
- Altered PBPs that reduce the affinity of the antibacterial agent for the target.
Bacterial impermeability or forced release may cause or contribute to bacterial resistance, particularly in Gram-negative bacteria.
The prevalence of resistance may vary geographically and temporally for individual species, and it is advisable to take local information on resistance into account, particularly when treating severe infections. If necessary, specialist advice should be sought if the local prevalence of resistance is such that the usefulness of amoxicillin, at least in some types of infection, is questionable.
Pharmacokinetics
Absorption
Amoxicillin completely dissociates in aqueous solution at physiological pH. It is rapidly and well absorbed when administered orally. The oral bioavailability of amoxicillin is approximately 70%. The time to reach maximum plasma concentration (Tmax) is approximately one hour.
The results of a pharmacokinetic study in which amoxicillin was administered at a dose of 250 mg three times daily in healthy volunteers on an empty stomach are presented below.
| Сmax | Tmax* | AUC(0-24 часа) | Т½ | Median (range) |
| (mcg/ml) | (time) | (μg h/ml) | (time) | |
| 3,3 ± 1,12 | 1,5 (1,0-2,0) | 1,36 ± 0,56 |
In the range of 250 to 3000 mg, bioavailability is linearly proportional to the dose (measured as Cmax and AUC). Absorption is not affected by concomitant food intake.
Hemodialysis can be used to eliminate amoxicillin.
Distribution
About 18% of the total amount of amoxicillin in plasma is bound to protein, and the apparent volume of distribution is about 0.3-0.4 l/kg.
After intravenous administration, amoxicillin is detected in the gallbladder, peritoneal cavity, skin, fat, muscle tissue, synovial and peritoneal fluid, bile and pus. Amoxicillin is not sufficiently distributed in the cerebrospinal fluid.
Animal studies have not shown reliable presence of metabolites in tissues. Amoxicillin, like most penicillins, can be detected in breast milk (see section "Use during pregnancy and lactation").
Amoxicillin has been shown to cross the placental barrier.
Biotransformation
Amoxicillin is partially excreted in the urine as inactive penicillic acid in amounts equivalent to 10-25% of the initial dose.
Elimination
The major route of elimination of amoxicillin is through the kidneys.
Amoxicillin has a mean half-life of approximately one hour and a mean total clearance of approximately 25 L/hour in healthy subjects. Approximately 60 to 70% of amoxicillin is excreted unchanged in the urine within the first 6 hours after administration of a single dose of 250 or 500 mg Amoxil. Various studies have shown that urinary excretion is 50-85% of amoxicillin within 24 hours.
Concomitant administration of probenecid slows the elimination of amoxicillin.
Age
The half-life of amoxicillin is similar for children aged 3 months to 2 years, older children and adults. Very young children (including premature infants) should not be given more than twice daily during the first week of life due to the immaturity of the renal elimination pathway. Since elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection and renal function should be monitored.
Gender
Gender does not significantly affect the pharmacokinetics of Amoxil when amoxicillin is administered orally to healthy men and women.
Renal impairment
The total serum clearance of amoxicillin decreases proportionally with decreasing renal function.
Liver failure
Amoxicillin should be administered with caution to patients with impaired liver function, and liver function should be monitored regularly.
Preclinical safety data
Preclinical data do not indicate any special hazard for humans based on safety pharmacology studies, repeated dose toxicity studies, genotoxicity studies, reproductive toxicity and developmental toxicity studies. Carcinogenicity studies with amoxicillin have not been performed.
Method of administration and dosage
- The dose of Amoxil is determined by the doctor, taking into account:
- The expected pathogen and its probable susceptibility to antibacterial agents
- The severity and localization of the infectious process
- The age, weight and state of renal function of the patient
- The duration of treatment should be determined by the type of infection and the patient's response to treatment and, as a rule, should be as short as possible.
Some infections require longer treatment.
In patients undergoing peritoneal dialysis
The maximum daily dose of amoxicillin is 500 mg/day.
Liver failure
Prescribed with caution with regular monitoring of liver function.
Method of administration
The risk is intended only for breaking to facilitate swallowing, and not for dividing into equal doses.
Amoxil is intended for oral use.
Food intake does not reduce the absorption of amoxicillin.
The tablet should be swallowed with water.
If the patient forgets to take the next dose of Amoxil, it should be taken as soon as possible.
Do not take a double dose to compensate for the missed dose!
Side effects
The most frequently reported adverse reactions are diarrhea, nausea and skin rash.
Adverse reactions reported from clinical trials and post-marketing surveillance of amoxicillin are presented according to system organ class and frequency.
The frequency of adverse reactions is divided into the following categories: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), frequency not known (frequency cannot be estimated from the available data).
Reporting Adverse Reactions
It is important to report suspected adverse reactions after authorisation of the medicinal product to ensure continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are encouraged to report any suspected adverse reactions to the medicinal product via the national adverse reaction and non-efficacy reporting systems.
If a patient experiences any adverse reactions, they are advised to consult a doctor. This recommendation applies to any possible adverse reactions, including those not listed in the package leaflet. You can also report adverse reactions to the Adverse Reactions (Actions) Information Database for Medicines, including non-efficacy reports. By reporting adverse reactions, you help provide more information on the safety of the medicine.
Contraindications
Hypersensitivity to the active substance, to any of the penicillins or to any of the excipients that make up the medicine.
History of severe immediate hypersensitivity reactions (eg, anaphylaxis) to another beta-lactam agent.
Overdose
Symptoms: gastrointestinal dysfunction – nausea, vomiting, diarrhea, which may result in water-electrolyte imbalance.
Crystalluria has been reported, sometimes leading to renal failure.
Patients with impaired renal function or those who have received high doses of amoxicillin may experience convulsions.
Treatment: induce vomiting or wash out the stomach, then take activated charcoal and an osmotic laxative. Maintain water and electrolyte balance. Amoxicillin is removed from the blood by hemodialysis. There is no known specific antidote.
Precautions
Hypersensitivity reactions
A careful review of previous hypersensitivity reactions to penicillins, cephalosporins or other beta-lactam agents should be made before initiating amoxicillin therapy.
Serious and sometimes fatal hypersensitivity reactions (including anaphylactoid and severe cutaneous adverse reactions) have been reported in patients receiving penicillin therapy. These reactions are more common in people with a history of penicillin hypersensitivity and in people with atopy. If an allergic reaction occurs, amoxicillin treatment should be discontinued and appropriate alternative therapy instituted.
Non-susceptible microorganisms
Amoxicillin is not suitable for the treatment of certain types of infections unless the pathogen is already documented to be susceptible or there is a very high probability that the pathogen will respond to amoxicillin treatment. This is particularly relevant when considering treatment for patients with urinary tract infections and severe ear, nose and throat infections. Convulsions
Convulsions may occur in patients with impaired renal function, or in patients receiving high doses of amoxicillin, or in patients with predisposing factors (e.g. history of convulsions, epilepsy or meningeal disorders.
Renal impairment
In patients with renal impairment, the dose should be adjusted according to the degree of impairment.
Skin reactions
The occurrence of febrile generalized erythema with pustules at the beginning of treatment may be a symptom of acute generalized exanthematous pustulosis. If this reaction occurs, amoxicillin should be discontinued and subsequent use is contraindicated.
Amoxicillin should be avoided if infectious mononucleosis is suspected, as the occurrence of a morbilliform rash has been associated with this disease after use amoxicillin.
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction has been described following amoxicillin treatment for Lyme disease (see Side Effects). It is directly related to the bactericidal activity of amoxicillin against the causative agent of Lyme disease, the spirochete Borrelia burgdorferi. It is manifested by fever, chills, decreased blood pressure, tachycardia, nausea, headache, muscle pain, worsening of existing or new symptoms of the underlying disease. The patient should be advised that this is a common phenomenon, usually resolves spontaneously, and is considered a natural consequence of antibiotic treatment for Lyme disease.
Overgrowth of non-susceptible organisms
Long-term use may occasionally result in overgrowth of non-susceptible organisms. Antibiotic-associated colitis has been reported with almost all antibiotics and may range in severity from mild to life-threatening. It is therefore important to consider this diagnosis in patients with diarrhea during or after taking any antibiotics. If antibiotic-associated colitis occurs, amoxicillin should be stopped immediately, a doctor should be consulted, and appropriate therapy should be initiated. Drugs that slow peristalsis are contraindicated in this situation.
Long-term therapy
During long-term therapy, periodic assessment of the functions of certain organs and systems is recommended: including renal, hepatic, and hematopoietic function. Increased liver enzymes and changes in blood counts have been reported.
Anticoagulants
There are isolated reports of prolongation of prothrombin time in patients receiving amoxicillin. When used in combination with anticoagulants, appropriate monitoring is necessary. To maintain the desired level of anticoagulation, dose adjustment of oral anticoagulants may be necessary.
Crystalluria
Crystalluria is very rare, mainly with parenteral therapy, in patients with reduced urine output. During the administration of high doses of amoxicillin, it is advisable to maintain adequate fluid intake and urine output to reduce the likelihood of amoxicillin crystalluria. In patients with urinary catheterization, patency should be checked regularly.
Interference with diagnostic tests
Elevated serum and urine amoxicillin levels may interfere with some laboratory tests. Due to the high concentration of amoxicillin in the urine, false-positive results are possible when using chemical methods.
When testing for glucose in urine during treatment with amoxicillin, it is recommended to use the enzymatic glucose oxidase method.
The presence of amoxicillin may interfere with the results of the estriol test in pregnant women.
Excipients
Amoxil contains less than 1 mmol (23 mg)/dose of sodium, i.e. it is practically sodium-free.
Use during pregnancy and lactation
Pregnancy
Animal studies do not indicate direct or indirect reproductive toxicity. Limited data on the use of amoxicillin during pregnancy in humans do not indicate an increased risk of congenital malformations. Amoxicillin can be used during pregnancy when the potential benefits outweigh the potential risks associated with treatment.
Breastfeeding
Amoxicillin is excreted in small amounts in breast milk with a possible risk of sensitization. Therefore, diarrhea and fungal infection of the mucous membranes are possible in a breastfed child, so breastfeeding may have to be discontinued. Use of amoxicillin during breastfeeding is possible only after benefit/risk assessment by a doctor.
Fertility
There are no data on the effect of amoxicillin on fertility in humans. Reproductive toxicity studies in animals have not shown an effect on fertility.
Effects on the ability to drive vehicles and operate machinery
No studies have been conducted on the effect on the ability to drive vehicles and other machinery. However, adverse reactions may occur, which may affect the ability to drive vehicles or other machinery.



